Abstract
Background
Proteasome inhibitors (PIs) are central to anti-MM therapy and 3 are currently licensed: bortezomib, carfilzomib and ixazomib, increasing treatment options. Bortezomib (BZ) and carfilzomib (CFZ) have been studied in head-to-head comparison using a CFZ dose of 56mg/m2 in doublet with dexamethasone in relapse (ENDEAVOR), and also at 36mg/m2 in triplet with melphalan and prednisolone in newly diagnosed non-transplant eligible patients. Differing results may relate to dosing and scheduling, as well as to different study populations. There is growing evidence for triplet regimens especially at relapse.
Aims
The MUK five phase 2 study compared the activity and safety of CFZ and BZ in triplet combination using a CFZ dose of 36mg/m2, with cyclophosphamide and dexamethasone (KCD vs VCD), for patients at first relapse, or refractory to no more than 1 prior line of therapy.
Methods
The study compares 8 cycles of VCD with 6 cycles of KCD (24 weeks treatment), and also assesses the benefit of maintenance carfilzomib in the KCD arm. Participants were randomised (R1) in a 2:1 ratio in favour of KCD, minimisation factors were β2M, prior bortezomib, prior ASCT and timing of first relapse (< or ≥12 months). Participants in the KCD arm with at least stable disease (SD) after 6 cycles of KCD were randomised (R2) 1:1 to receive maintenance carfilzomib or no further treatment. Participants in the VCD arm did not receive maintenance. Inclusion criteria included Hb>80g/L, neutrophils>1.0x109/L, platelets 50x109/L and GFR>30ml/min.
KCD therapy was 28 day cycles of biweekly carfilzomib 20/36mg/m2 IV (weeks 1-3) while VCD was 21 day cycles of biweekly bortezomib 1.3mg/m2 SC (weeks 1 and 2), both with cyclophosphamide 500mg orally weekly (weeks 1-3 only for KCD) and dexamethasone 40mg orally weekly. Co-primary endpoints were ≥VGPR rates at 24 weeks post R1 (powered for non-inferiority comparison, deemed non-inferior (NI) if 90% confidence interval (CI) for odds ratio (OR) >0.8), and PFS from R2 (superiority). Disease response was assessed according to the Modified IWG Uniform Response Criteria, and minimal residual disease (MRD) by multiparameter flow cytometry (10-4).
Results
From Feb 2013 to Sept 2016, 300 participants were randomised, 201 to KCD and 99 to VCD. Patient and disease features were balanced between arms, median ages 67 and 69 years, 57.5% and 64.6% male, 93.5% and 94.9% ECOG 0-1 for KCD and VCD respectively. Median time from diagnosis was 32.5 and 36.1 months, 50.0% and 45.5% were ISS 2/3, and 66.2 and 67.7% had had an ASCT.
While 81.6% of patients in the KCD arm received all 6 treatment cycles, only 53.5% in the VCD arm received all 8 cycles; reasons for stopping treatment were toxicity (KCD, 7%; VCD, 19.2%), disease progression (6.5%, 6.1%) and withdrawal of consent (2.5%, 11.1%). Dose modifications occurred in 78.6%, and 85.4% of patients in the KCD and VCD arms respectively.
A total of 196 and 96 patients were evaluable for efficacy analysis in the KCD and VCD arms. Major response (≥VGPR) at 24 weeks for KCD and VCD was 40.2% and 31.9% respectively (OR 1.48; 90% CI (0.95, 2.31), deemed NI). Overall response (≥PR) was 84.0% and 68.1% (OR 2.72; 90% CI (1.62, 4.55); p=0.0014, deemed superior). MRD negativity (all evaluable patients; n=134 KCD, n=48 VCD) at 24 weeks was 16.4% for KCD and 12.5% for VCD.
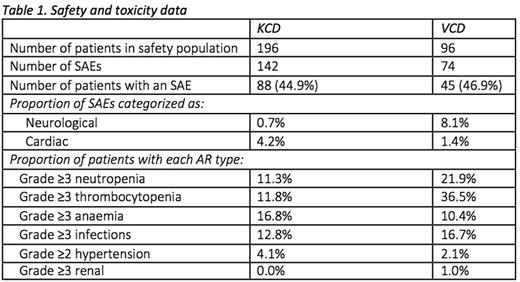
The safety population was 292 patients (KCD, 196; VCD 96). Treatment emergent neuropathy occurred in 21.4% and 56.3% of patients in the KCD and VCD arm, respectively. The proportion of patients with grade ≥3 neuropathy or grade ≥2 neuropathy with pain (key secondary endpoint) was lower with KCD (1.5%, vs 19.8% with VCD; p<.0001). Details of serious adverse events (SAEs) are given in Table 1; these were largely comparable between the arms, except for more neurological SAEs in the VCD arm (8.1% vs 0.7%) and more cardiac SAEs in the KCD arm (4.2% vs 1.4%). Adverse reactions (ARs) were also comparable except for more grade ≥3 neutropenia and thrombocytopenia with VCD and more grade ≥3 anaemia with KCD.
Conclusion
Major response (≥VGPR) to KCD therapy is non-inferior to VCD and overall response rate is superior to VCD over a fixed treatment duration. This may be related to better tolerability and reduced incidence of neurotoxicity with KCD, with superiority of KCD in terms of grade ≥3 neuropathy or grade ≥2 neuropathy with pain. Further details on safety and activity will be presented at the meeting.
Yong: Janssen: Honoraria, Research Funding; Amgen: Honoraria, Research Funding. Auner: Amgen: Honoraria, Research Funding. Williams: Janssen: Honoraria, Other: travel support, Speakers Bureau; Celgene: Honoraria, Other: travel support, Speakers Bureau; Takeda: Honoraria, Other: travel support, Speakers Bureau; Amgen: Honoraria, Speakers Bureau; Novartis: Honoraria. Cavenagh: Amgen: Honoraria; Janssen: Honoraria; Celgene: Honoraria. Kaiser: Chugai: Consultancy; BMS: Consultancy, Other: Travel expenses; Takeda: Consultancy; Janssen: Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria. Rabin: Novartis: Consultancy, Speakers Bureau; Takeda: Consultancy, Other: Travel support for meetings, Speakers Bureau; Celgene: Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Janssen: Consultancy, Other: Travel support for meetings, Speakers Bureau. Ramasamy: Janssen: Honoraria; Takeda: Honoraria, Research Funding; Amgen: Honoraria. Garg: Janssen: Other: travel support, Research Funding, Speakers Bureau; Takeda: Other: travel support; Novartis: Other: travel support, Research Funding. Hawkins: janssen: Honoraria. Morgan: Takeda: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Bristol Myers: Consultancy, Honoraria. Davies: Amgen: Consultancy, Honoraria; Bristol-Myers: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Consultancy, Honoraria. Owen: Celgene: Consultancy, Honoraria, Research Funding; Takeda: Honoraria, Other: travel support; Janssen: Consultancy, Other: travel support.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal